Water purity is extremely important in the pharmaceutical and biochemistry, dissolved or suspended particles, organic compounds, impurities and other contaminants preventing use tap water in laboratory and scientific research. Parameters used as resistivity, conductivity, particle size and concentration of microorganisms for water quality and therefore specify the intended uses for water. Some applications can tolerate the presence of certain impurities in the water, but others, such as high performance liquid chromatography (HPLC) require remove most contaminants.
Pollutants
Water is an excellent solvent and is available in virtually any place on Earth. This property makes is free of any contamination.
- Particles: silt and sediment can be removed by passing the water through filters of 10 to 20 microns (or less if necessary).
- Microorganisms: bacterial agents are a real challenge for water purification systems. Their growth rate, size and design require effective resistance (detection, incoming water withdrawal, growth inhibition, etc..). Bacteria are measured in colony forming units per millimeter and can be removed by disinfectants. As a result, their secretions and cell fragments also be removed to prevent contamination.
- Endotoxins, pyrogens, ribonucleic acid and deoxyribonucleic: bacterial cell fragments and derivatives. Harmful to tissue culture. Detectable by LAL test (limus amoebocyte lysate).
- Dissolved inorganic elements: include phosphates, nitrates, calcium and magnesium, cargono dioxide, silicates, iron, chlorine, fluorine and any other natural or artificial chemical product due to exposure to the environment. Electrical conductivity (μSiemens / cm) is used to monitor concentrations of ions, and the resistivity (milliohm / cm) is used to identify the ions present in small concentrations. These contaminants influence the water hardness and alkalinity / acidity.
- Elements dissolved organic pesticides, residues or fragments of plants and animals. Analyzers are used total organic carbon (TOC) to measure the CO2 emitted by bodies subject to oxidation. The water free of organic matter is used primarily in applications in which an analysis of organic substances such as high performance liquid chromatography (HPLC), chromatography, and mass spectrometry).
Scientific applications require removing certain types of contaminants. Furthermore, the production of pharmaceuticals requires, in most cases, the almost total elimination of impurities (local regulatory agencies / international standards or criteria determine specific).
Purification Process
There are different methods that are commonly used in water purification. Its effectiveness depends on the type of pollutant treated and the type of application you are going to use the water:
- Filtering: This process therefore consist of any of the following:
- Filtering coarser particles also called filtering, can be used from a sand filter of 1 mm to a cartridge filter of 1 micron.
- Microfiltered: uses Device 1 to 0.1 microns to filter bacteria. A typical implementation of this technique is in the brewing process.
- Ultrafiltration: removes pyrogens, endotoxins and DNA fragments and ADR.
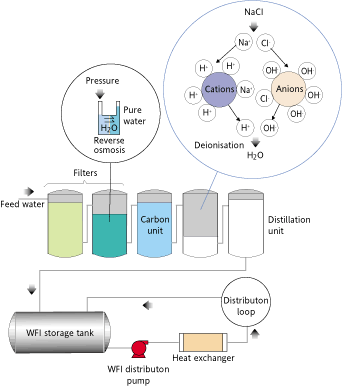
- Reverse osmosis (RO): Reverse osmosis is the finest level of liquid filtration. Instead of a filter using a porous material which acts as a sieve to separate unidirectional molecular size particles.
- Distillation: the oldest method of purification. It is a cost effective method, but can not be used for a process demand. The water must be distilled and then stored for later use, so you can re-contaminated if not stored properly.
- Activated carbon adsorption: acts as a magnet for chlorine and organic compounds.
- Ultraviolet radiation: with a certain wavelength, can sterilize bacteria and other micro-organisms decompose.
- Deionization: also called ion exchange is used to produce purified water demand by passing through resin layers. The negatively charged resin (cation) removes positive ions, whereas the positively charged resin (anion) removes negative ions.Supervision and ongoing maintenance cartridge produces the purest water.
Sterilisation with hot water
The water purification equipment can be sterilized with hot water appropriately combining the exposure time and temperature. A significant use of this process is the deactivation of viable microbes. It is noteworthy that endotoxin reduction is not achieved as a direct result of the sterilization process with hot water.
Depending on the source water, the system operating conditions and operating procedures and maintenance of the end user may be required conventional chemical cleaning procedures.
The hot water sterilization involves the inclusion of heat exchangers in a system of cleaning in place (CIP) traditional gradually heating and cooling the water circulating through the system of reverse osmosis membranes. The membrane manufacturers typically require heating and cooling speed controlled to protect the membrane and irreversible damage to ensure long-term operation of the system.
A sequence of common hot water sterilization comprising the following phases:
- Initialization (check conditions).
- Warming.
- Retention.
- Cooling.
Therefore, the control system should provide flexibility in the way of achieving precise control and repeated sterilization environment and include the following features:
- Precise control loop with programmable setpoints profiles.
- Sequential Control for sterilization.
- Operator messages displayed.
- Pump control active / reserve.
- Secure data collection on-line system for analysis of purified water.
- Local operator screen with sharp graphics and controlled access to parameters.
The system Eycon ™ Visual Supervisor is a perfect solution for this application.
Originally posted 2013-03-24 16:33:52.